Stem cell therapy has in recent years emerged as a potential alternative to invasive surgery, medication, and numerous other conventional approaches to patient care. Mesenchymal stem cells (MSCs) are particularly promising due to their relative abundance in adults, ease of harvesting, relatively minimal side effects, and effectiveness across a host of chronic illnesses.
Against this backdrop, there has been a steadily increasing body of research pointing to the immunomodulatory properties of these cells – that is, their ability regulate the immune system. Given these discoveries, MSCs have unsurprisingly received great attention as an effective therapeutic response to acute diseases such influenza, and the novel coronavirus (COVID-19).
MSCs can reduce the effects of this cytokine storm by releasing their own cytokines that counteract inflammation, drowning out the signal of harmful cytokines sent by the lungs. In previous clinical investigations, a portion of MSCs delivered intravenously were found to migrate to the lung, when the lung was not the targeted site for therapy. Once seen as a drawback, this unintended collection of MSCs in the lung is a key advantage to reducing inflammation and promoting tissue healing in the lungs of COVID-19 patients.
Additionally, MSCs are known to lack the cell surface receptors Angiotensin-converting enzyme 2 (ACE2) and Transmembrane protease, serine 2 (TMPRSS2) that allow for the entry of the coronavirus into the cell. Thus, MSCs administered to patients cannot be infected by the coronavirus, and will therefore not “add fuel to the fire.”
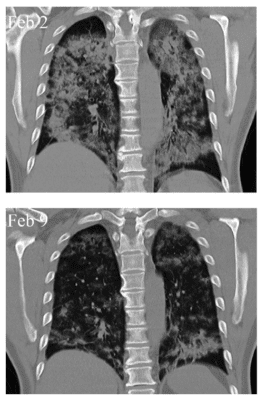
As of May 1, 2020, there were more than 30 clinical trials in progress investigating the use of MSCs as a treatment for COVID-19 pneumonia symptoms. The first and most notable of these studies, conducted by an international coalition of researchers from China, United States, India, France and other countries, appeared in Aging and Disease. All seven patients reported significantly improved symptoms within two days of MSC treatment and showed decreased cloudy appearance of the lungs that indicative of pneumonia severity (see image)
Another recent case study of a 65 year old woman in critical condition due to COVID-19 provides additional support to the evidence from the previous study. The patient had undergone treatment involving antiviral drugs, steroids, antibiotics, and immunotherapy, with limited benefit. She then underwent three rounds of MSC administration. Following the second round of therapy, significant improvements were seen in the following:
- body temperature, oxygen saturation, and other vital signs
- levels of pro-inflammatory proteins as measured in several blood tests
- white blood cell counts
- healthy organ function
Further, the patient was able to switch from invasive ventilation to a nasal cannula, and experienced improvement in overall mobility.
These studies demonstrate that MSC therapy may provide great efficacy and safety, with fewer side effects than those associated with those drugs and biologics under clinical investigation. It is important to note that none of the recipients of stem cell therapy in any COVID-19 study has reported negative side effects due to the treatment. This approach merits further attention, given the intense virtually unprecedented challenges posed by the COVID-19 pandemic.
Learn More From the Pioneer in Stem Cell Research
We encourage you to contact us, to learn more about how MSCs may provide benefit to patients suffering from diverse diseases – including those impacting lung function. Please complete this brief contact form, or reach out via phone ((844) 446 7827) or email ([email protected]).
